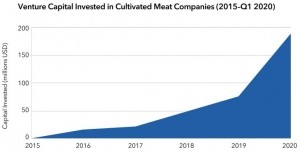
'Not only viable, but inevitable...' Cell-cultured meat in the spotlight as UPSIDE Foods opens 'most advanced cultivated meat production facility in the world'

So when will it start producing meat?
With construction of the 53,000 sq ft facility and innovation center now complete, UPSIDE Foods could go to market tomorrow from an operational perspective, but is working with regulators to secure approval before launching its first commercial products in the US, Eric Schulze, PhD, VP product and regulation, told FoodNavigator-USA.
“The formal agreement lays out the process,” said Schulze, who was speaking to us ahead of today’s grand opening at the site, which can produce 50,000lbs of finished product a year, with a future capacity of over 400,000 pounds per year – a drop in the ocean in the context of conventional meat production, but a significant milestone for an industry that didn’t even exist six years ago.
“FDA under its existing authority, will evaluate the safety of the product and the process as it would for any food product or new ingredient through a voluntary consultation process,” he said.
“Once the agency has no further questions regarding the safety of our product, it passes that determination along to USDA, if it's meat or poultry, where the USDA will use that determination to perform its suitability assessment as it would for any meat or poultry ingredient,” added Schulze, who noted that the facility includes a quality assurance room where products are tested to ensure safety and an office for federal inspectors to oversee the process.
“Then, the assessment forms that basis of judgment for issuing a grant of inspection for the facility and approval for any label to be put on packages,* again, just like any new meat product.
“So we continue to actively engage with both agencies on all aspects of this process. We are using the formal agreement, which again, is a voluntary consultation process, so any ingredient in there that qualifies as a food additive under FDA’s, existing authority will be evaluated as such under existing regulation.”
Transparency and cell-cultured meat
But how transparent is this process? Will there be a dossier or something a bit like a GRAS notification that the public can view to learn more about the process by which UPSIDE Foods and others in the space are producing their cell-cultured meat?
According to Schulze, who used to work at the FDA before joining UPSIDE Foods (then known as Memphis Meats) in 2016: “It is my understanding that FDA intends to disclose on its website details pertaining to pre-market safety consultations on cultivated meat, poultry, and seafood products, and UPSIDE Foods fully supports that, if indeed it pursues that option.”
He also welcomed news that the FDA plans to publish draft guidance on the pre-market consultation process, adding: “As a former regulator myself, I can tell you that we were always eager to provide draft guidance to new fields because predictability and clarity in regulation helps everybody.
“The entire industry, including our regulators, is committed to transparency because we must earn consumers’ trust.”


‘A lot of the assumptions from the pharmaceutical industry have not been challenged’
So what still needs to change to bring down the cost of growing animal cells outside of animals, which proponents of the technology argue is inherently more efficient as resources are spent on growing only the cells that make up the meat product rather than maintaining the day-to-day activities of an entire animal’s body?
According to Schulze, UPSIDE Foods has already made significant progress in reducing the cost of growth media and finding efficiencies in bioprocess scale up: “Those continue to be the two largest levers for reducing cost.”
However, he did not provide details: has it identified cheaper ways to produce the most expensive components in the growth media? Is it working with cell lines that require fewer inputs? Has it found ways to recycle media, or found other efficiencies that give investors confidence that this technology is commercially viable at scale?
He added: “We have an extremely strong conviction that this is not only a viable industry, but that this is an inevitable industry.”
‘The pharma world has never felt pressure to decrease cell manufacturing costs or increase scale beyond existing systems’
Asked about the recent article in The Counter (drawing upon two techno-economic analyses of cell-cultured meat: CE Delft 2021 and Humbird 2020) arguing that cell-cultured meat faces "intractable technical challenges at food scale,” he said: “Scientific discourse is an incredibly healthy aspect of any developing field, and it’s incredibly important that we have constructively critical dialogues.
"We're cognizant of all the risks laid out in the Humbird paper, and we have an extraordinary team working to de-risk them, and we've made remarkable progress in doing so."

But he added: “Prior to our company, no entity had focused on cell manufacturing at low cost and at a very large scale, so as such, it's easy to take conservative assumptions from the pharmaceutical world.
"The pharma world has never felt pressure to decrease cell manufacturing costs or increase scale beyond existing systems, so our progress today gives us strong conviction that we can surpass the pharmaceuticals industry cell manufacturing systems in terms of cost and scale.”
“We continue to challenge these long held assumptions that surround large scale cell culture that were built primarily in the biopharmaceutical industry, while acknowledging the fundamental tenets of biology and evolution as our technical guideposts.”
‘Our current products do not contain any synthetic scaffolds’
Asked if the first products from UPSIDE Foods will be hybrids featuring a combination of plant protein and cell-cultured meat – an approach Eat Just is deploying in Singapore with GOOD MEAT and New Age Meats says it plans to deploy both for cost and textural reasons, he said: “We are exploring all processing methods for meat, poultry and seafood products.”
But he clarified: “I want to make a point about scaffolding [which some companies are using to co-culture cells around in a tissue engineering approach, and others are adding post-production via extrusion to add texture]. The cells we use produce their own matrix, so we do not have any need for any manmade scaffolds at this time, although we are exploring a range of product formulations that include scaffold materials that would be edible, but our current products do not contain any synthetic scaffolds.
“There are ways of growing cells where you require a substrate for those cells to attach and form a tissue. But we are relying upon the inherent genetic programming of these cells using their naturally occurring processes already inside of them to produce the tissues and attach to each other.”
‘A product agnostic platform’
With respect to IP, he said, “We aim to lead in all categories, and we see ourselves as a leader not only because we were the first to fully attempt commercialization and scale of cultivated meat, but also because we have a product agnostic platform that can produce any type of meat and on top of that, building products for the future that explore what meat can be.”
Asked about the ecosystem that is beginning to form around cell-cultured meat (third parties developing bioreactors, cell lines, growth media, scaffolding etc), he said: “We have a really healthy competitive ecosystem which is incredible, given this field is only six years old.”
From a growth media perspective, however, UPSIDE Foods has an entire team of people that develop media in-house, he said.
“That is important we think competitively, so we can build a supply chain for media components, which is significant portion of the [cost of the] production process, that we can then tailor to particular cells or products, which gives us a particular advantage.”
UPSIDE Foods – which has recently hired former PepsiCo executive Amy Chen as COO, and industrial biotech veteran Dr. Bob Kiss as EVP Technology – confirmed the recent departures of co-founder Nicholas Genovese and VP process development KC Carswell (who is understood to have left the company after moving to Australia). Schulze did not provide further comment.
*The USDA has issued an advance notice of proposed rulemaking (ANPR) to solicit comments on how to label cell-cultured meat and poultry as startups in the space edge closer to commercialization, but says it will review labels submitted before the rulemaking process is completed on the understanding that they may need to be changed down the road to comply with its final regulations. Read more HERE.
- Watch UPSIDE Foods founder and CEO Dr Uma Valeti at today's opening ceremony.

Vaporware?
The UPSIDE Foods facility is opening following the publication of a lengthy article in The Counter arguing that cell-cultured meat faces "intractable technical challenges at food scale,” echoing comments made by Impossible Foods founder Dr Pat Brown, who has described cell-cultured meat as "vaporware" in a recent interview.
However, startups in the nascent field have challenged the notion that the obstacles to industrial-scale cell-cultured meat production are insurmountable, and argued that the Humbird paper relies on publicly available information that does not reflect recent advances in the field.
They also acknowledge that higher-density cell cultures, more efficient use of food-grade media, food-grade equipment in facilities, and dramatic cost reductions in recombinant protein and growth factor production are needed for this technology to be commercially viable at scale.
GFI: 'We’d like to be the first to know if cultivated meat were incontrovertibly a futile exercise...'
The Good Food Institute - a nonprofit promoting plant-based, fermentation-based, and cell-cultured meat - has addressed some of the issues raised in The Counter's article in a post on its website, noting that, "Modeling a process on public data from parallel but distinct cell culture processes that are also necessarily out of date (biopharma companies are typically not publishing their most recent innovations on process or cell line improvements, preferring to retain them as intellectual property) will lead to a muddled understanding of the state of the art, which can be particularly misleading in such a rapidly-progressing field."
It added: "GFI has no vested interest in any particular alternative protein technology. We support innovations that have the greatest potential for impact, and we’re strongly motivated to not divert precious resources toward endeavors that seem clearly incapable of success — we’d like to be the first to know if cultivated meat were incontrovertibly a futile exercise!"
'Governments must invest in these innovations now'
Founder and CEO Bruce Friedrich added this morning: "In 2016, UPSIDE Foods was the first cultivated meat company to raise a Seed round. Flash forward five years, and now there are more than 70 companies working to create consumer-ready cultivated meat products.
"But there is still too much innovation required for a single company, or even a handful of companies, to truly transform our food system. Governments must invest in these innovations now if we’re to stand a chance of moving the world to net-zero emissions by 2050."
Cell-cultured meat vs conventional meat
Supporters of cell-cultured meat claim it is better for animals and the environment, but also offers other advantages over traditional meat in that it does not contain bacterial pathogens that pose food safety risks.
Also, they claim, it will not suffer from price/supply volatility risks from animal infectious diseases (avian flu, porcine epidemic diarrheal virus); it requires fewer inputs for a given quantity of meat; and is more controllable and tunable, enabling production of only high-grade meats in quantities dictated by consumer demand, rather than by the biology of the animal.
And while antibiotics may be used in laboratory settings given the number of researchers handling cells, key players say they are confident that commercial-scale production of cultured meat will be possible without antibiotics, potentially a key selling point given consumers’ concerns about the use of antibiotics in the conventional meat industry and its contribution to antibiotic resistance.
The regulatory pathway for cell-cultured meat in the US
Under a joint agreement announced in March 2019, the FDA will oversee cell collection, cell banks, and cell growth and differentiation, with a transition to FSIS (USDA) oversight to occur during the cell harvest stage. FSIS will then oversee the production and labeling of meat and poultry derived from these cells.
An FDA spokesperson told FoodNavigator-USA in August that it “intends to issue draft guidance on the pre-market consultation process,” adding: “The FDA is actively working in this space and consulting with a number of companies interested in bringing cultured animal cell food products to the US market.
"We cannot comment on the status of individual consultations that are in progress. However, FDA intends to communicate publicly once the FDA consultation is complete.”
Read more about the labeling and nomenclature for cell-cultured meat HERE