Proposed ‘Office of Food Safety Reassessment’ would target chemicals that entered food supply through GRAS process
Rep. Jan Schakowsky (Ill.) and Rep. Rosa DeLauro (Conn.) reintroduced June 7 the Food Chemical Reassessment Act of 2023, two years after introducing the first version of the bill, which would require FDA to create an Office of Food Safety Reassessment to regularly review the safety of chemicals that entered the food supply chain through legal “loopholes” or which were reviewed by FDA decades ago.
Research by the Environmental Working Group found the vast majority of new food chemicals added to the food supply between 2000 and 2022 did so via the Generally Recognized As Safe (GRAS) provision, which the Representatives argues was initially proposed in 1997 to allow “clearly safe ingredients like vinegar” to enter the food supply – not the “thousands of chemicals added to food to make it last longer, taste better and look more enticing.”
By creating an Office of Food Safety Reassessment, the legislation would enable, and require, FDA to study every three years the safety of at least 10 chemicals added to food or food packaging, according to the Representatives.
Topping the list of chemicals they specifically want FDA to review are Tert-butylhydroquinone (TBHQ), titanium dioxide, potassium bromate, perchlorate, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), brominated vegetable oil (BVO), propyl paraben, sodium nitrite and sulfuric acid.
The bill would also reestablish a Food Advisory Council to direct FDA on how best to review the chemicals and identify future chemicals for review.
“Food safety is a second-class citizen in the Food and Drug Administration. An Office of Food Safety Reassessment is desperately needed, particularly as we face ongoing food safety challenges due corporate negligence and dysfunction at the FDA,” Rep DeLauro said in a statement.
Rep. Schakowsky agreed, noting, “It’s time to put the ‘F’ back in FDA, and this bill is an important step in ensuring the foods we eat are safe and free from harmful chemicals.”
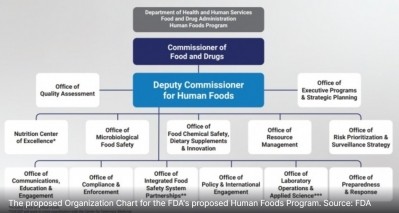
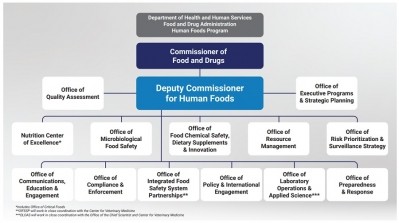
Reimaging FDA's structure
Their sentiments echoed those expressed by many when an independent review of FDA conducted by the Reagan-Udall Foundation called on the agency to reorganize so that food safety was more prominently featured and supported.
FDA Commissioner Robert Califf has said he would heed the foundation’s recommendation and appoint a single leader of a new Human Foods Program, but some food industry stakeholders and advocates worry the agency is moving too slowly and doing the least amount possible to appear responsive to the scathing report.
As such the current legislation proposed by Schakowsky and DeLauro has garnered applause from the EWG, Breast Cancer Prevention Partners, Food & Water Watch, Mamavation, Center for Environmental Health, PIRG, Earthjustice and others.