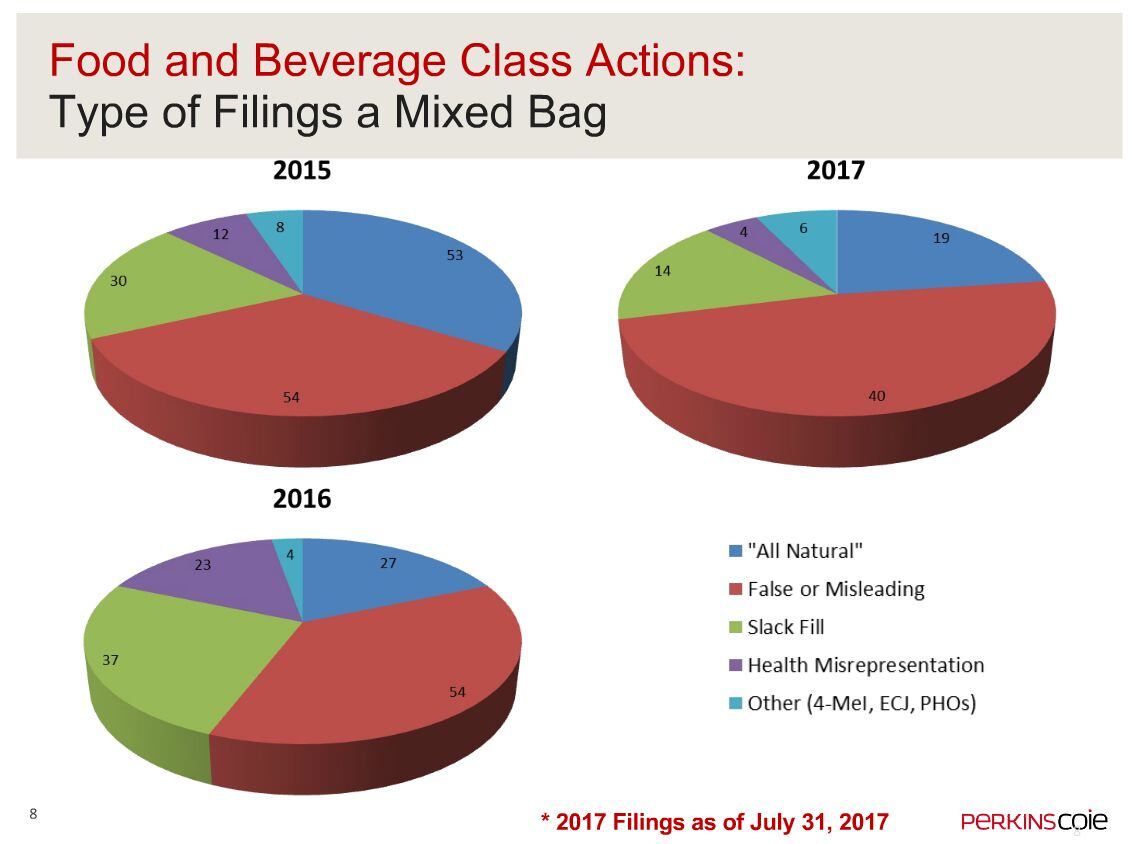
Speaking at a mid-year food litigation summary webinar hosted by the law firm earlier this month, Perkins Coie partner Charles Sipos said: “All natural litigation dipped a bit in 2016, but in 2017 there’s been a bit of a resurgence [19 cases in the first seven months of the year vs 27 for all of 2016] and if the current trend continues, we’re expecting about a 30% increase in the filings of these cases in 2017 as compared to 2016.”
Plaintiff's attorneys in more recent 'natural' cases have adopted new strategies, targeting everything from pesticide residues on ready-to-eat cereals labeled as ‘100% natural’ (General Mills), to incidental synthetic additives used in natural flavors that do not, according to the plaintiff, belong in beverages labeled as ‘all-natural’ (Hint Inc), to ‘natural’ cheese using milk from cows treated with rbST (Sargento), and ‘natural’ yogurts made with milk from cows likely given feed from GM crops (Dannon).
How are courts treating the new ‘all-natural’ lawsuits?
While some 'natural' lawsuits have been stayed on primary jurisdiction grounds following the FDA’s 2015 call for comments on natural claims, “a couple of courts have lifted stays or refused to grant them because of how long the FDA has been considering comments that have been submitted,” noted Sipos.
For example, the judge handling the lawsuit over Hint Inc’s use of propylene glycol (an incidental additive commonly used in natural flavors) in beverages labeled as ‘all-natural,’ recently refused Hint’s request to stay the case, arguing that, “Without any sense as to when, or even if, the FDA will follow up on its request for public comment and issue formal guidelines regarding the use of 'natural' in food labeling, it would be improper for the Court to stay the proceedings in this action.”
'Reasonable' consumers and pesticide residues
In the pesticide residue lawsuits, there has been better news for defendants, although some cases are still proceeding through the courts, said Sipos.
“Some initial rulings suggest that this attempt to equate natural with purity is not one that courts are particularly persuaded by.”
In a July 12 ruling tossing out a group of lawsuits against General Mills querying ‘natural’ claims on Nature Valley products containing trace levels of the herbicide glyphosate, for example, Judge Michael J. Davis said it was “implausible that a reasonable consumer” would believe that the products “could not contain a trace amount of glyphosate that is far below the amount permitted for organic products.”
Sipos added: “The plaintiff's bar continues to be pretty creative in coming up with new ways of attacking natural claims on products, but at least initially on these glyphosate cases, you see some resistance by courts as to whether that misleads consumers.”
Could the FDA be forced to act on ‘natural claims?
As for the FDA, which has maintained radio silence on the ‘natural’ issue since its November 2015 call for comments on natural claims (the comment period closed in May 2016), pressure could be brought to bear by Congress, said Sipos.
In a July 17 report from the [House] Committee on Appropriations on a bill making appropriations for agriculture, rural development, the FDA, and related agencies for fiscal year 2018, the committee calls on the FDA to report to Congress on the status of its rulemaking on 'natural' claims 60 days after the bill’s passage.
“The Committee directs FDA to provide a report within 60 days of enactment of this Act on the actions and time frame for defining ‘natural’ so that there is a uniform national standard for the labeling claims and consumers and food producers have certainty about the meaning of the term.”
According to Sipos at Perkins Coie, “That line item encourages the FDA to actually promulgate a regulation in order to have some sort of uniform, standard for the use of the term [natural]…
"We’ll be tracking that bill closely, but in the meantime, it shows that Congress agrees with a regulatory approach to this issue versus a litigation case by case approach.”
Natural still resonates, even if its meaning is elusive

When it comes to ‘natural’ claims, while many FoodNavigator-USA readers doubt whether it retains much value, a surprisingly high percentage – 76% - in our 2017 reader survey agreed that ‘the word ‘natural’ still resonates strongly with consumers on food & beverage product labels,’ even though “educated consumers will always look past it to the back of the label.”
One reader explained: “I think that people are ascribe a lot more meaning to it than is actually guaranteed,” while another conceded: “Yes, 'natural' still resonates, even though everyone has their own definition of what it means.”
