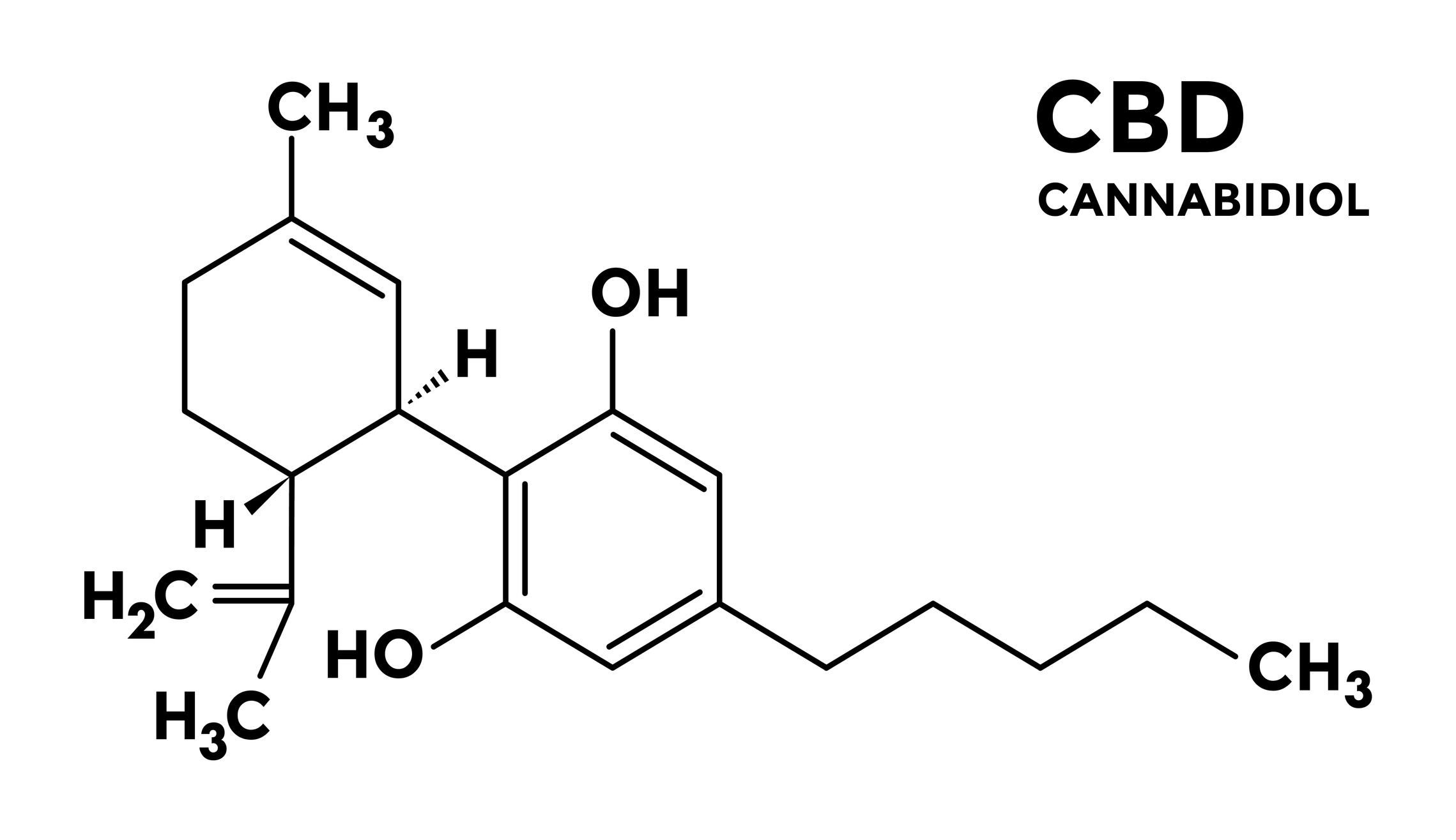
The company’s synthetic CBD is structurally and stereochemically identical to CBD chemically extracted and isolated from plants, Jim Mish, President and CEO of Purisys, told NutraIngredients-USA. “They are – in effect – nature-identical,” he said.
In addition to CBD, Purisys also produces over 50 major and minor cannabinoids relevant to pharmaceutical and consumer products, as well as degradants, metabolites and analytical reference standards, said Mish. In addition to CBD, more than 30 other cannabinoids have also been descheduled.
“Today, as a community, we in the cannabinoid business are running at breakneck speed toward the starting line of a race that has a chance to do a great deal of good for people’s lives,” said Mish.
“And, while there are still a great number of issues that need to be addressed before the market has a chance to really grow, I am confident of a few things: Quality and purity are going to be essential if we are going to create safe, efficacious and legal products that are trusted by consumers.
“Great formulations and excellent consumer experience must be there for both initial product acceptance and long-term viability. And that in the cannabinoid space, multiple manufacturing technologies – pharma-based, biosynthetic, grow and extract - will have their place and can even work together.”
Food, beverages, and supplements
When asked if the company is eyeing the food, beverages, and supplements spaces, Mish said the answer is a “resounding yes”.
“We anticipate a great deal of opportunity in these channels as the regulatory situation regarding cannabinoids gets sorted,” he added.
On the regulatory considerations, Mish said the company’s goal is be “recognized as the global market and thought leader for advanced cannabinoid ingredients and solutions that are safe and efficacious. We are known for delivering the highest standard of purity and quality and we have the capacity to support major commercial brands.”
Their cannabinoids are produced in accordance with cGMP (ICH Q7 and applicable sections of 21 C.F.R. Parts 210 and 211), he added.
“Regardless of whether we are planning a self-affirmed GRAS/ New Dietary Ingredient status, we want to develop a fair and honest dialogue with the FDA as well as all key regulatory and market stakeholders – and to support innovations that create new ways to contribute to human health.”
Odorless and tasteless
On pricing, Mish said that Purisys, which was formed as a spinoff of a leading pharmaceutical API company, would be extremely competitive with all CBD ingredients in the market, including hemp extracted cannabinoids. “This will be particularly important in food and beverage markets,” he added.
The company’s patent-protected manufacturing process produces a high-purity CBD (less than 0.001% THC), which is both odorless and tasteless, said Mish.