Leafreport.com recently shared results from its comprehensive review of CBD products that they tested throughout 2020-2021.
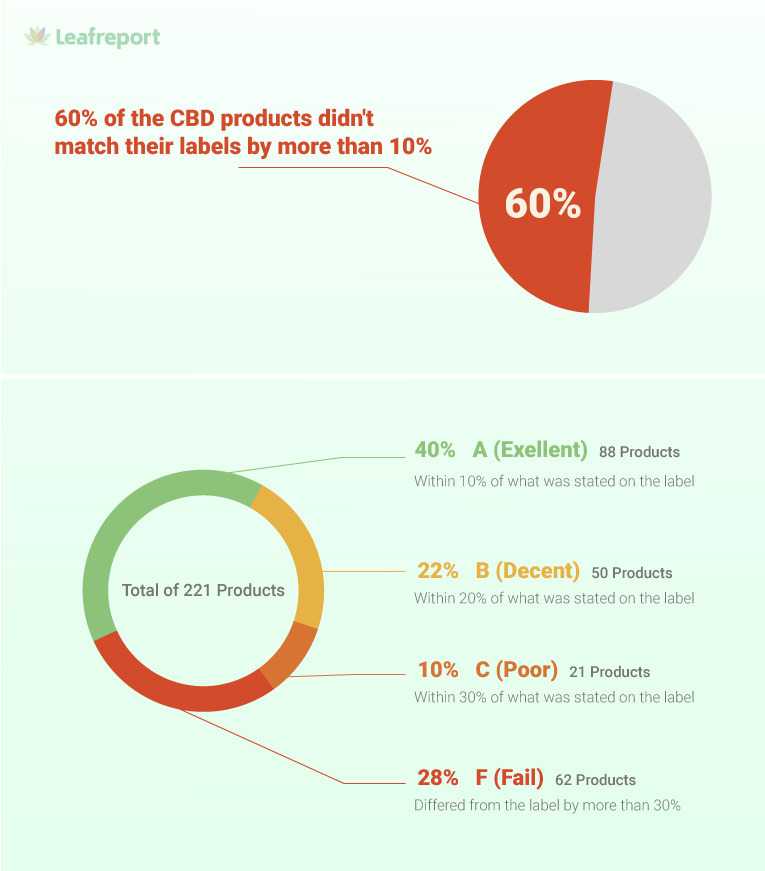
According to the findings, 60% of the CBD products sent for testing were more than 10% off of the stated label amounts.
How it works
Leafreport measures the accuracy of CBD products by comparing their labeled CBG strength to the amount found by the third-party lab tests. They base this accuracy on cannabinoid levels that are within 10% of what the label indicates.
Throughout 2021, the peer-reviewed watchdog sent 221 CBD products from 111 brands for third-party testing. These included 35 CBD oil, 40 topical, 40 edible, 22 beverage, 55 pet, as well as 29 coffee and tea products. In order to receive an A grade, products needed to be within 10% of the labeled CBD content. Products that were off from the label by a higher percentage received a B, C, or F grade.
“Our mission was simple: send the products for third-party testing and compare the results to the amount and type of CBD listed on the product label,” said Lital Shafir, the head of product at Leafreport. “Leafreport’s goal is to help promote transparency across the CBD industry and educate consumers so they can access products that are safe and offer the contents being advertised. Reports like this show that the industry still has a long way to go in terms of meeting acceptable standards for consumers. We hope that reports like this help educate those who want to use CBD and make them more informed in their purchasing decisions.”
Findings
The report revealed that 28% of products received the worst (F) grade for having CBD levels that differed from the label by more than 30%. On average, the CBD content of the products was off from the label by nearly 25%. Beverages had the worst results, with only 18% of products matching the label and two products containing no CBD at all. Out of 97 products advertised to contain broad or full-spectrum CBD, 44% were mislabeled. In the beverage, topical, and edible categories, more products received an F than an A.
“The results were pretty surprising,” said Gal Shapira, product manager, Leafreport. “We believed that there would be some mislabeling of some of the products, but never expected that the vast majority of them would be mislabeled. It’s interesting to see how many products on the market simply do not contain the amount of CBD that they think they do, whether it’s too little or too much. There was even a product that contained 221% of what they advertised.”
Shapira also told NutraIngredients-USA that although the number isn’t necessarily high, about 44% of pet products matched the labeled CBD strength. Shapira said the team expected this number to be much lower.
Categories
“We found that oils were the most accurate product group we tested with 74% matching the labeled CBD strength and an average difference of only 13.5% from the label. Beverages had the worst results, with only 18% of products matching the label and two products containing no CBD at all," explained Shapira.
In a previous interview, Shafir told us that in general, it's usually easier to get accurate dosing for oils and tinctures compared to other products, so you can see the majority of the "A" scored products are tinctures and oils. “CBD beverages are difficult to formulate and contain relatively small amounts of CBD, which means that variations of even a few milligrams can have a big effect," she said.
Foot-dragging on CBD
In April 2019, FDA formed a CBD Policy Working Group. Since then, the agency has issued a few non-binding guidance documents related to CBD products. However, no regulations have been set forth, with the agency citing the need for more research.
“The differences can be attributed to a lack of testing. Because the US Food and Drug Administration (FDA) is still reviewing information about the safety and potential benefits of CBD, it makes it more difficult to regulate products within the industry. Additionally, regulations can vary by country which only adds to the issue. Due to this inconsistency throughout the industry, companies often aren’t required to complete full testing and there isn’t an entity enforcing that they do so. This is why we recommend that companies complete third-party testing to facilitate transparency about their product contents," said Shapira.
With legislation such as the Safe Banking Act of 2021, the Hemp and Hemp-Derived CBD Consumer Protection and Market Stabilization Act of 2021 and the Hemp Access and Consumer Safety Act, Congress has the authority to address key CBD issues.
In July, a bill was put forth by Senate Majority leader Chuck Schumer, D-NY, that offered a legal pathway for CBD supplements and directed FDA to set safe upper exposure limit.
Sports & Active Nutrition Summit to focus on CBD
In the sports nutrition sphere, advocates say CBD can help with recovery, manage post exercise inflammation and even help ameliorate the effects of traumatic brain injury. But what does the science actually say about this popular ingredient? Expert speakers at the upcoming Sports & Active Nutrition Summit, scheduled for Feb. 14-16 in San Diego, will delve into this question as well as the trends in product formulation. Speakers will also explore the pitfalls in finding secure, quality sources of raw material. Attendees will gain insights on what challenges these brands face and how best to serve their needs from a supplier perspective.




