While the peanut allergy health claim is an important step, it’s only part of the story, explained Before Brands CEO and co-founder Ashley Dombkowski, PhD. Over 75% of people with a food allergy are allergic to something other than peanut and food allergies affect an estimated 6 million children in the US, or about two kids per classroom.
The SpoonfulOne powder blend, which launched at the start of October, is said to be the only product to cover the complete set of foods that make up 90% of food allergies. It includes gentle portions of peanut, milk, tree nuts, egg, fish, shellfish, wheat, soy and sesame seeds, along with 400 IU of vitamin D for immune balance.
SpoonfulOne is labeled as a dietary supplement, but the company does have plans to go beyond supplements, Dombkowski told us.
Early introduction and measured delivery
The product platform was born out of research by Dr Kari Nadeau, a pediatrician, mother of five, and the director of the Sean N. Parker Center for Allergy and Asthma Research at Stanford University. Beyond Brands has licensed Dr Nadeau’s IP relating to the formula as the base of SpoonfulOne, explained Dombkowski.
The product formula remains proprietary and the doses have not been revealed, but the formula is based on a couple of key points, said Dombkowski. “First and foremost we wanted something that would be safe, and the product is developed to support safe feeding, but with meaningful exposure levels for the complete set of foods that cause most food allergies. It’s not about maximizing the dose, it’s about a broadly safe dose in non-allergic populations.”

SpoonfulOne comes packaged in pre-measured daily single-serving packets to stir into children’s foods from the age any time after solid foods have been introduced, making it convenient, portable and easily contained, she explained. There is no minimum duration that children should be fed the product, explained Dombkowski, because scientists just don’t know yet.
For example, the landmark LEAP study [New England Journal of Medicine, 2015, Vol. 372, pp. 803-813] found that early intro of allergic foods (peanut) reduced risk of allergy to that food at age 5 by 80%. “Think of it like exercise,” said Dombkowski. “The earlier you start it, the better.”
Safety data
Health Claim
The US Food and Drug Administration issued a new qualified health claim in September that reiterates and reassures parents of new advice to give 4- to 10-month-olds products containing peanut to reduce their risk of developing an allergy to the ingredient.
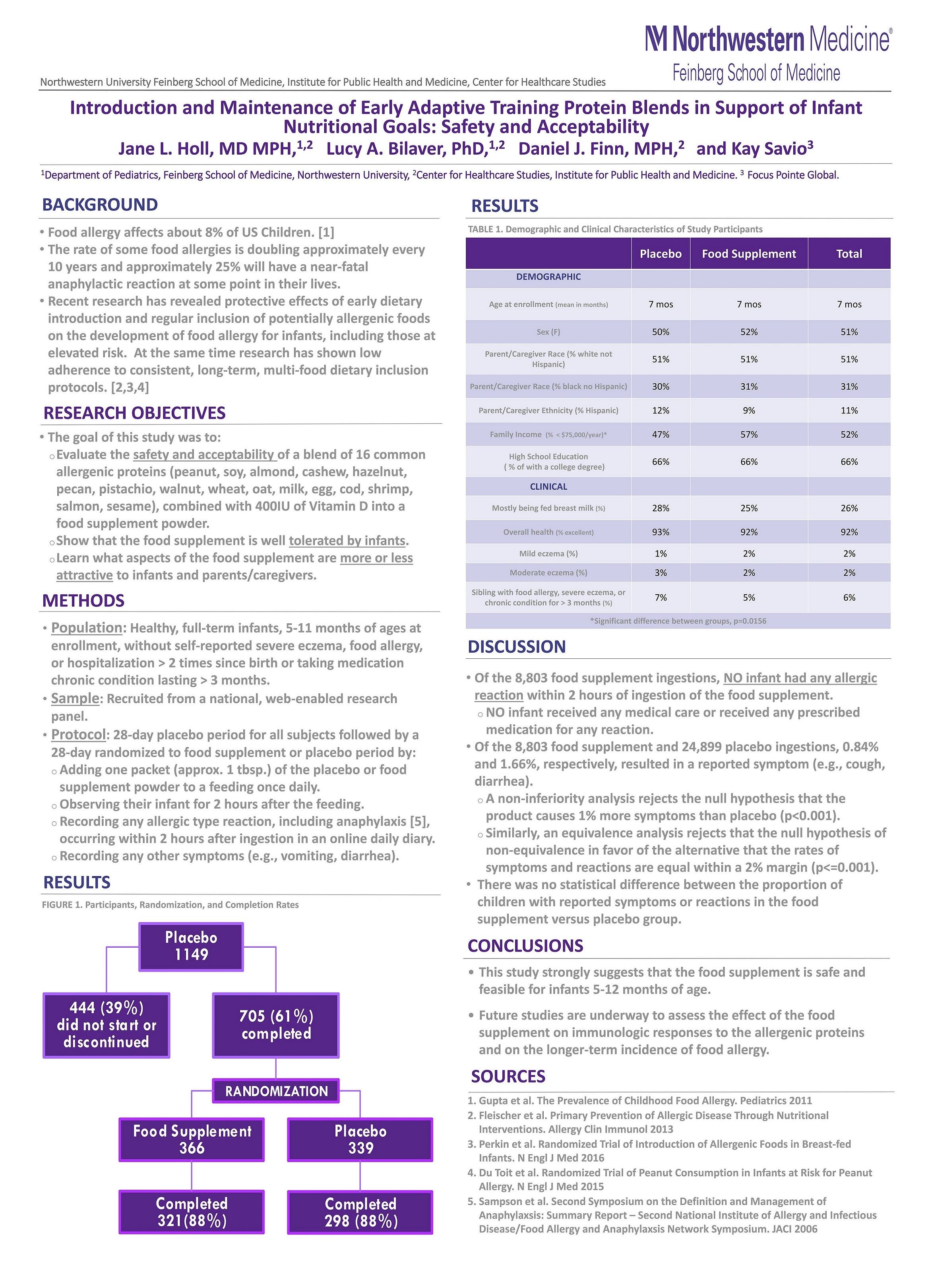
The safety of the product is supported by data from a randomized, blinded, placebo-controlled nationwide independent study led by Jane Holl, MD, MPH, from the Northwestern University Feinberg School of Medicine. Dr Holl recently presented her findings at the Pediatric Asthma and Allergen Meeting in London (see poster below).
The “Introduction and Maintenance of Early Adaptive Training protein blends in support of Infant Nutritional Goals: safety and acceptability" (the I’M EATING study) included 700 full-term infants, 5-11 months of age without reported severe eczema or diagnosed food allergy.
The data showed that in over 8,800 feedings of SpoonfulOne, no infant was reported to have had any sign or symptoms of an allergic reaction. The product was well-tolerated in real-life, at-home settings.
Dr Holl commented: “The safety and tolerability of SpoonfulOne shown in this study provide highly reliable data for parents and pediatricians seeking scientifically robust findings in support of the benefits of feeding infants multiple, potentially allergenic foods contained in SpoonfulOne early and consistently.
“Given the 88% study completion rate, it is clear that SpoonfulOne was easy for parents to incorporate into their infant’s regular feeding routine, which is encouraging given the adherence challenges that often arise when parents attempt to consistently feed their infant a range of foods.”
Future studies are underway to assess the effect of this supplement on immunologic responses to the allergenic proteins and the longer-term incidence of food allergy, Dombkowski told us.
Availability
The product is currently available only through its website by subscription, which is aimed to boost adherence. The company does have plans to expand distribution channels over time, said Dombkowski, and will explore international markets like the UK and Australia when the time is right (but it has not issued any guidance on when that may be).
Despite being early days, Dombkowski said she is pleased with the early consumer response. “Pediatricians have been looking at how to get patients and families to start these foods and keep them in the diet, and our safety data means a lot to them,” she said. “We’ve also seen a good response from the parents, with them sharing recipes they’ve developed to vary the ways they are mixing the products in with foods, from frozen pancake teething sticks to shepherd’s pie. There’s also been a lot of interest from parents of infants with older children with a food allergy who are looking to introduce the foods to the infants without jeopardizing the older child. Our stick pack helps.”
The product is designed for children who have not been diagnosed with a food allergy.
The rise in food allergy
According to a 2013 study by the Centers for Disease Control and Prevention (CDC) the incidence of food allergies in children in the United States increased 50% in the period from 1997 to 2011. By some estimates, 15 million Americans struggle with food allergies, and 1 in 13 children are affected. And the annual health care cost for these conditions is around $25 billion. The problem is not restricted to the US; the same assessment estimates that 17 million people in Europe deal with food allergies, too.

