To determine the total protein content in food materials, it is typically necessary to determine a measurement of the total nitrogen content in the sample first, and calculate protein using a factor specific to the sample matrix. While the measurement (or determination) of nitrogen content can be performed using a classical wet chemistry method, in recent years the combustion-based Dumas method has gained popularity.
“While an instrumental method typically means there are more up-front costs, laboratory productivity and throughput are greatly increased,” said Mason Marsh, Organic Product Manager with LECO Corporation. “With the combustion method, results can be returned in minutes, rather than the hours experienced with wet chemistry methods. In addition, the ease-of-operation and overall safety characteristics of performing an analysis with a combustion method lead to improved operator health and safety as well as greater satisfaction.”
Combustion instruments performing total nitrogen determination use a high-temperature furnace in an oxygen environment to combust, or burn, the sample. The combustion gases are then swept from the furnace, scrubbed, and processed to eliminate various components that would interfere with the total nitrogen result. Nitrogen combustion gases, in the form of NOx, are reduced to N2 prior to detection using a Thermal Conductivity (TC) detector.
One limitation to combustion instruments, however, is that the sample mass used for analysis is limited to the amount capable of the instrument. For most food samples, this does not present an issue, as a nominal sample size under 0.5 grams is adequate. However, some samples containing lower levels of nitrogen (such as dry corn starch, Neutral Detergent Insoluble Nitrogen (NDIN) filter bags, low level filtrate, beer, low level filtration media, etc.) can present a challenge for analysis.
How can the combustion method be optimized to accommodate these lower-level nitrogen samples? “One way is to increase the sample mass, therefore increasing the amount of nitrogen presented to the instrument for detection,” said Marsh. “This can also be significantly helpful when analyzing samples that are heterogeneous in nature as well.” But does increasing the sample size affect the precision of the results?
Recently, a series of analyses including starches, beer, nutritional drinks, and other low level nitrogen materials tested this theory utilizing the FP828 and FP928 Nitrogen/Protein Determinators (LECO Corporation, St. Joseph, MI). Both instruments are combustion instruments capable of returning an analysis of nitrogen. While the FP828 is capable of analyzing samples under 1.0 gram, the FP928 is designed for larger samples over 1.0 gram (typically as much as 2.0 or even 3.0 grams). Both instruments operate in a similar theory, though the FP928 has a different furnace design resulting in advantages for these larger samples. The similarities in their abilities to accommodate two different types of sample sets make these two instruments ideal for performing nitrogen analysis for a wide range of samples. First, let’s take a look at how each instrument operates.
For the FP828, the analysis process looks something like this:
- A sample weighing under 1.0 gram is weighed into a container and transferred to a sealed purge chamber. Atmospheric gas surrounding the sample container is removed.
- Sample is dropped into the furnace in a pure oxygen environment to ensure complete combustion.
- Combustion gases are swept from the furnace, moisture is removed, and the gasses are collected in a ballast.
- The gases equilibrate and mix within the ballast before an aliquot of the gas is extracted and introduced into a flowing stream of inert gas for analysis.
- A heated reduction tube, filled with copper, is used to convert NOx to N2 and to remove excess oxygen. Carbon dioxide (CO2) is removed by LECOSORB®, and water is removed by Anhydrone®.
- The aliquot gas is carried to a TC Cell to detect nitrogen (N2).
For the FP928, the sample analysis process is nearly identical, with the exception of the furnace design and sample introduction process. With this instrument, a larger sample, weighing between 1.0 and 2.0 grams, is weighed into a sample “boat”. The sample boat is then transferred to a horizontal furnace specially designed for the atmospheric gas purging and combustion of larger samples. The open design of the crucible sample boat eliminates any atmospheric gas interference by purging gas surrounding the sample boat and from the interstitial space within a granular solid sample, providing an additional benefit to the analysis of samples with low levels of nitrogen.
Results of Analysis
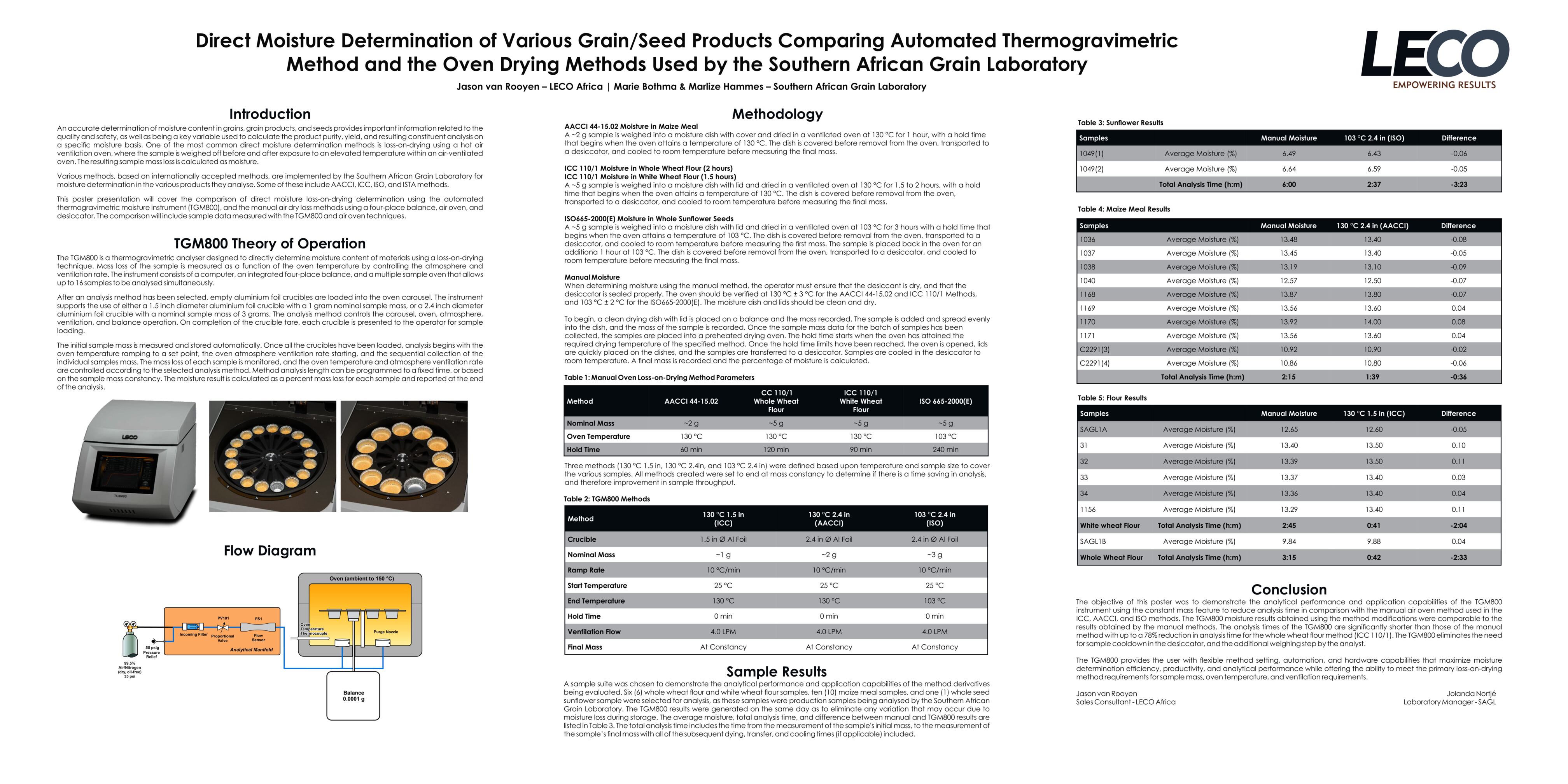
The results pictured below show the standard deviation between varying sample masses for different types of powder and liquid materials. The average percentage of nitrogen determined is shown under each sample.
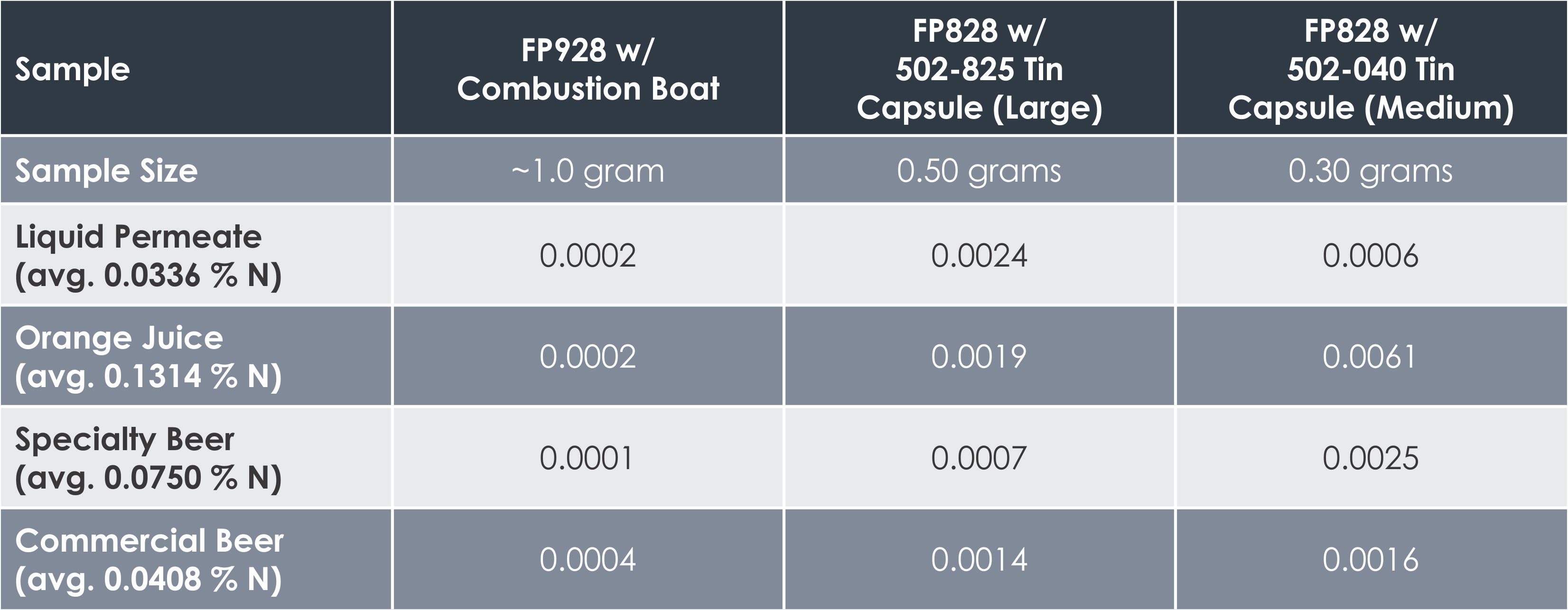
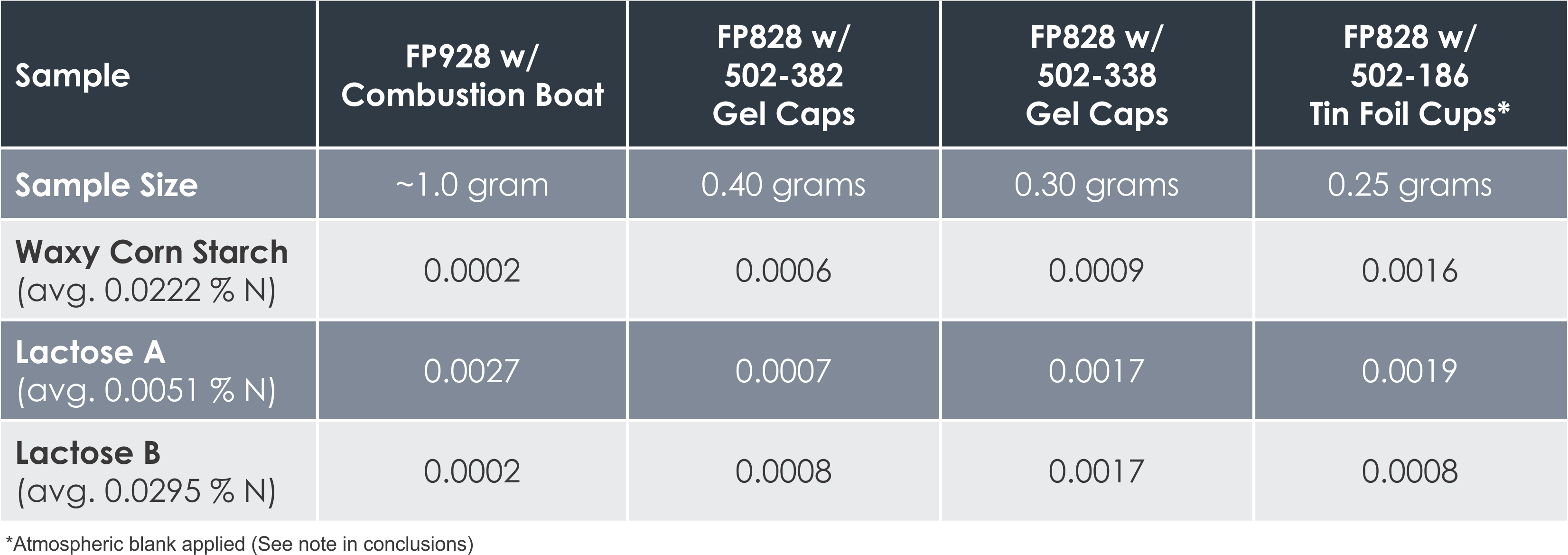
“In these results we can see that improved precision was generally obtained in the analyses utilizing a larger sample mass, with the FP928 typically returning the most precise results,” said Fred Schultz, Analytical Chemist with LECO Corporation. Some variation can be expected due to sample inhomogeneity that is common with certain materials.
“Samples analyzed on the FP828 in the Tin Foil Cups experienced decreased precision due to the probable influence of the atmospheric blank,” Schultz continued. This occurs when some atmosphere is trapped within the sealed sample container when it is encapsulated in the tin foil cup, causing biased nitrogen results at low nitrogen concentrations. Because of this, an atmospheric blank should be determined for the FP828*. An atmospheric blank is not necessary with the FP928 due to the open design of its sample crucible boats.
“For all liquid samples, the FP928 demonstrated superior precision due to the larger sample masses analyzed,” Schultz said.
Contact us to view the complete analysis parameters, procedures, and results of this analysis.
Conclusion
While analyzing materials containing low levels of nitrogen can be somewhat challenging when utilizing the combustion method for the analysis of protein, establishing optimization parameters and procedures that include the analysis of a larger sample mass can help. For liquids and powders containing low concentrations of nitrogen, increasing the sample’s mass improves precision for these samples. Powder sample inhomogeneity and the need for an atmospheric “blank” correction added additional steps to the analysis process for the FP828.
"Utilizing the FP928 returned more precise results, without the extra steps and uncertainty involved with an atmospheric blank correction,” said Marsh. “This simplifies the analysis process for the operator.”
See how the FP828 and FP928 Nitrogen/Protein analyzers can transform your total protein analysis.
*Atmospheric Blank Procedure
- Analyze reagent grade sucrose (finely ground) several times using similar masses of sucrose to the mass of samples being analyzed.
- Enter the actual mass of sucrose.
- The nitrogen value obtained is considered the atmospheric blank, and can be automatically compensated for when using the FP828 software.