Published in Cell, the mouse study suggests that certain microbes shape their hosts' metabolism very early in life and that disrupting, for example through exposure to antibiotics, during infancy can cause metabolic changes that appear to increase the risk of obesity in adulthood.
Led by Martin Blaser from the NYU Langone Medical Center, the research team said that their findings provide further steps in identifying which gut bacteria are crucial to metabolic health - adding that such information could be used to help restore levels of those helpful microbes and promote healthy metabolism in adulthood after an infant encountered microbiome disruption due to illness or from medical treatments such as antibiotics.
"We identified infancy as a critical window where host metabolism is especially vulnerable to microbiota disruption with antibiotics," said Blaser. "This highlights a need for judicious use of antibiotics in clinical practice in early life."
The team also noted that the research characterises important variables in early-life microbe-host metabolic interaction and identifies several taxa consistently linked with metabolic alterations.
Study details
Blaser and his colleagues noted that it has been previously suggested that the microbes which begin to colonise the gut at birth can be easily disrupted during infancy - and that this can have a long-term effect on weight. Indeed, farmers in the USA have been taking advantage of this phenomenon for decades by exposing livestock to low doses of antibiotics to promote growth.
Building on this, the research wanted to examine whether the exact timing and duration of early exposure to antibiotics could make a difference, as well as identify specific bacteria that may protect against the potentially harmful health effects. To address these questions, they gave long-term, low-dose penicillin treatment to two groups of mice: 4-week-old mice after weaning and mouse mothers beginning shortly before their pups' birth.
They found that earlier penicillin exposure led to more substantial obesity in adulthood and worse metabolic health, especially in males. Early exposure to antibiotics also reduced levels of several types of potentially protective bacteria and exacerbated the effects of a high-fat diet on obesity, said the team.
Indeed, in a separate experiment, the researchers found that only 4 weeks of antibiotic exposure beginning shortly before birth was sufficient to produce obesity, which lasted well after penicillin treatment ended.
"These temporal changes indicate that low-dose penicillin suppresses multiple taxa that typically peak early in life. Although high-dose (therapeutic) antibiotic exposures reduce microbiota diversity in animals and humans, low-dose penicillin increased phylogenetic diversity at 4 weeks, which is consistent with decreases in particular prominent taxa and detection of taxa with lower representation," wrote the researchers. "By sampling across time, we identified microbes that were altered prior to the development of adiposity, suggesting roles in host metabolic development."
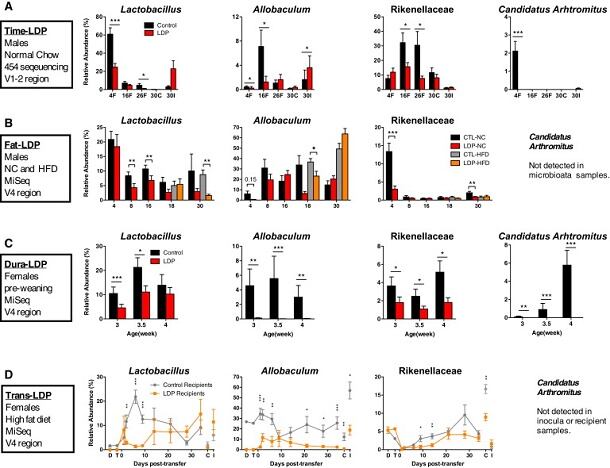
Furthermore, Blaser and his colleagues noted that it is the alteration of our gut microbiota, rather than use of antibiotics per se caused these metabolic changes:
"Our findings imply that restoring good bacteria could prevent the long-term metabolic effects of early antibiotic exposure," explained Laura Cox, first author of the study.
"We identified four candidate bacteria that may be metabolically protective, and we're working on follow-up studies to determine if we can prevent weight gain by giving these bacteria back following antibiotic therapy."
Source: Cell
Volume 158, Issue 4, 14 August 2014, Pages 705–721, doi: 10.1016/j.cell.2014.05.052
"Altering the Intestinal Microbiota during a Critical Developmental Window Has Lasting Metabolic Consequences"
Authors: Laura M. Cox, Shingo Yamanishi, et al
