In a letter to the GAO dated March 28, DeLauro – who serves on the agriculture appropriations subcommittee – said: “I am writing to request that GAO investigate what regulatory framework, if any, exists for cell-cultured food products and how this framework compares to other international approaches.
“While not yet commercially available, the potential introduction of this new type of product into the nation’s food supply and economy raises many important questions. For example, the US Cattlemen’s Association recently filed a petition with the USDA to exclude from the definition of meat any products not derived directly from animals. In addition, at least one dairy group has stated it believes the use of the word ‘milk’ for animal-free products creates confusion in the minds of consumers and thus should be limited only to products derived from animals.
“On the other hand, producers of cell-cultured foods argue that the products are safe and more efficiently produced than meat or milk products from live animals.”
Right now, she claimed, “It remains unclear exactly how cell-cultured food products should be regulated.”
Jurisdiction
USDA is responsible for ensuring that meat, poultry, eggs and fish sold in the US are safe and correctly labeled, while the FDA* is responsible for ensuring that all other food products are safe and properly labeled, she said. The FTC, in turn, monitors advertising.
“More information is needed for Congress to address this emerging sector in the United States and to ensure it is properly overseen by the relevant agencies once these products are commercially available. In light of the above, I would like to request conduct a comprehensive review of the following:
- What unique challenges, if any, exist in overseeing the safety of cell-cultured foods in the United States?
- What regulatory framework and labeling requirements, if any, exist in the United States to oversee cell-cultured food products, and to what extent, if any, have relevant agencies begun preparing for the commercialization of cell-cultured foods?
- How do other countries, such as Canada, the European Union, and/or Japan, oversee cell-cultured foods?"
What is meat?
Asked about jurisdiction in a recent interview about how cultured meat should be labeled, attorney Rebecca Cross (formerly with Davis, Wright, Tremaine LLP and now with Outermost, an incubator for plant-based and cell-cultured food companies launching later this year), said:
“The USDA has two regulatory definitions of ‘meat,’ essentially one for purposes of labeling, and another for purposes of inspections. My reading of these regulations is that if the USDA is determined to have jurisdiction, clean meat falls within the definition of ‘meat’ for purposes of labeling, but not for the purposes of inspections, which are geared toward slaughtered animals.”
Asked about labeling, she said: “The USCA is essentially seeking to require that animals be slaughtered in order for companies to use the term ‘meat,’ when consumers are – more and more – seeking products that are slaughter and cruelty-free. I would be surprised if the USDA acts on this petition.”

Speaking at our FOOD VISION USA conference last year, GFI senior scientist Dr Liz Specht said cultured meat offered several advantages over traditional meat:
- It does not contain bacterial pathogens that pose food safety risks
- It has a longer shelf life
- It will not suffer from price/supply volatility risks from animal infectious diseases (avian flu, porcine epidemic diarrheal virus)
- It requires fewer inputs for a given quantity of meat
- It is "more controllable and tunable," enabling production of only high-grade meats in quantities dictated by consumer demand, rather than by the biology of the animal.
GFI: Meat doesn't have a standard of identity
As cultured meat is still ‘meat’ (animal fat, muscle and connective tissue cells grown in vitro instead of invivo), there’s no clear legal reason why companies should not describe it as such, provided labels are truthful and not misleading, added Jessica Almy, director of policy at the Good Food Institute (GFI).
As for what ‘meat’ is, she said: “Meat doesn't have a standard of identity. Clean meat is real animal meat that's grown outside of an animal, so it would make sense to call it meat. That said, clean meat producers will want to communicate the benefits of their products to consumers, so it's highly unlikely they'll sell it in a plain cellophane package with only the word ‘meat’ on the label.”
As for meat producers – some of which (Cargill, Tyson) have recently invested in cultured meat and plant-based meat brands – the North American Meat Institute (NAMI) has not taken a formal position, “though we do have questions on how the products will be regulated,” Eric Mittenthal, VP of public affairs, told us.
“Regarding lab grown, animal based products, such products do not appear to conform to the definition of ‘meat’ as provided in the USDA regulations [9 CFR 301.2] and might better fit the definitions of either ‘meat food product’ or ‘meat by-product’ also in the USDA definitions”
What's the path to commercialization?
Technically, as cultured meat is a whole food and not a food additive, no pre-market approval would be required, although this would be product dependent.
However, given the novelty of the process and the scrutiny pioneers will face, companies in the space are working closely with the FDA as they approach commercialization, and may put together GRAS determinations (which they can submit to the FDA in the hope of receiving a letter of no objection), predicted one industry source.
GFI: Clean meat is similar to conventionally-produced meat in nutritional respects, but the process does not involve slaughter and the accompanying health risks
The Good Food Institute says it does not have a firm opinion on what the regulatory structure should be for clean meat, but stresses that "Transparency is a central value for everyone working in the field. In contrast, modern farms and slaughterhouses are closed to the public, and many are pushing unconstitutional 'ag-gag' laws to prevent people from seeing what happens on factory farms."
Meat, as it is currently produced, is a leading cause of food poisoning illness and death, claimed the GFI: "This is simply not a concern for clean meat.... These facts should be taken into consideration when determining what regulations clean meat should meet. Clean meat is similar to conventionally-produced meat in nutritional respects, but the process does not involve slaughter and the accompanying health risks.
“Clean meat eliminates the risk of microbial and drug residue contamination, a major public health benefit. Given that clean meat will have these and other significant societal benefits, including using resources more efficiently than conventional meat, as well as a lower carbon footprint, the regulatory path to market should ensure consumer safety and confidence without being onerous for producers."
FDA: 'It's reasonable to think cultured meat could be consumed safely'
The FDA is not commenting on labeling, but said it was “committed to supporting innovation in the food supply,” and encouraged manufacturers to “engage with us to address any questions they may have.”
A spokesperson added: “Given information we have at this time, it seems reasonable to think that cultured meat, if manufactured in accordance with appropriate safety standards and all relevant regulations, could be consumed safely.”
*USDA has primary jurisdiction over meat, poultry, and certain egg products, but co-ordinates with the FDA where appropriate to determine responsibility for specific food products.
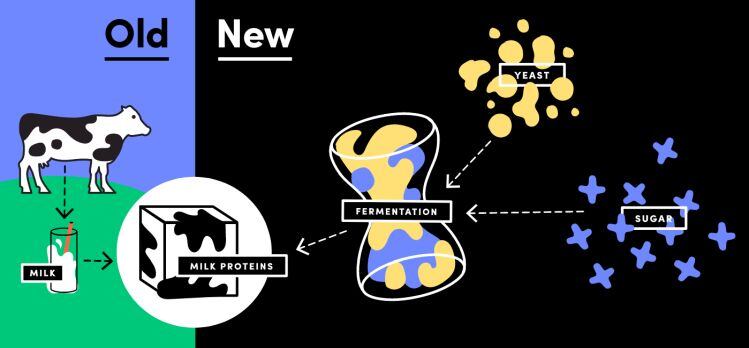
ANIMAL FREE DAIRY? What is the regulatory status of Perfect Day’s 'animal-free' dairy ingredients, which deploy genetically engineered yeasts that have been ‘programmed’ to produce dairy proteins without cows?
It’s new territory, acknowledges co-founder Ryan Pandya - who has been in conversations with the FDA almost from day one and is working on a GRAS determination for the ingredients - but it’s actually not as complicated as you might think, given that Perfect Day is making ‘dairy’ proteins (albeit using a different process) that are well understood and widely consumed.
As for the term ‘milk,’ a term that has a standard of identity, discussions are ongoing, but the FDA’s primary concern is that labeling is not misleading, he contends.
“Consumers demand and deserve transparency so we want to make sure that it’s clear that it’s dairy protein, but that it is animal free, and therefore suitable for vegans.”
