“The Center for Food Safey and Applied Nutrition (CFSAN) has always been my biggest customer, shall we say, because they do not have an ombudsman,” like other FDA centers, FDA Chief Ombudsman Laurie Lenkel told industry stakeholders last week during a webinar hosted by the Alliance for a Stronger FDA.
“I have lobbied for one probably with every Center Director for the past 20 years,” she added, explaining that she helps stakeholders across industry and FDA agencies – including those with center-level ombudsmen who do not report to her but often work collaboratively with her team – to resolve challenges and restore trust.
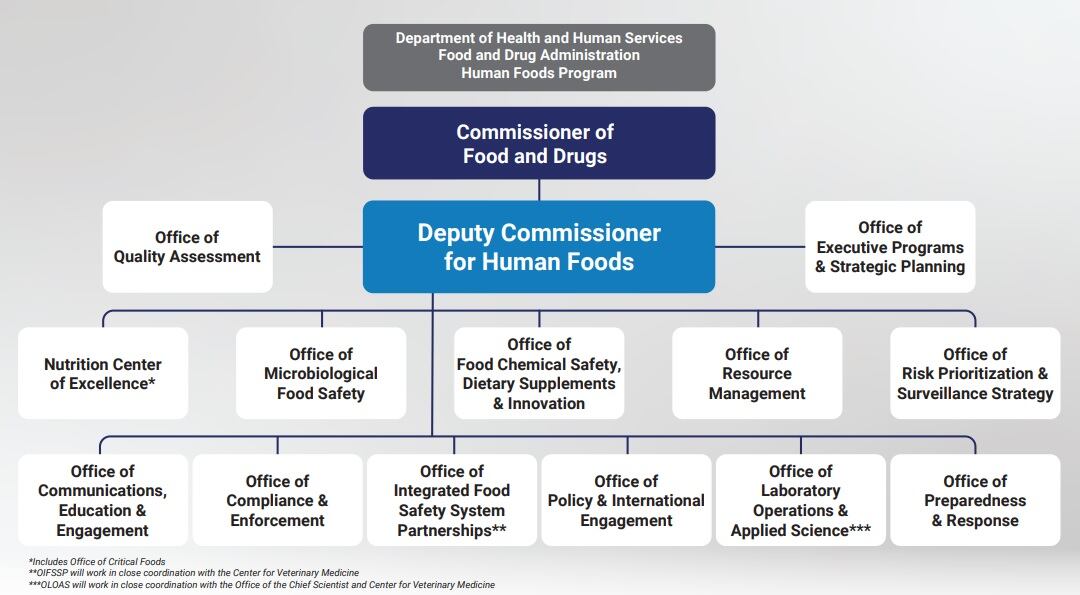
However, she explained appointing an ombudsman for CFSAN would help given the vast majority of the more than 400 cases that her team of three tackles annually fall under CFSAN’s jurisdiction and are “very diverse.”
“CFSAN deals with products, such as dietary supplements that can border on drugs, as well as all types of food and nutrition issues,” and despite 20 years in her role Lenkel said she often hears requests for which she doesn’t know the answer. And while she said she is always eager to research and find an answer or path forward, she also said she believes that the appointment of an ombudsman under the new Human Foods Program or for CFSAN “will help coordinate some efforts a little better.”
Lenkel said she and the ombudsman for the Office of Regulatory Affairs “have already devised something to help promote [the appointment of an ombudsman for CFSAN and the Human Foods Group], and we would be happy to speak to [Deputy Director Jones] about the need for the program.”
She also noted that industry could lobby the new deputy director, who will take the helm Sept. 24.
Ombudsman could ‘spur’ greater communication, transparency, collaboration, but would not be a panacea
While appointing an additional ombudsman to CFSAN or the Human Foods Group could help stakeholders more quickly identify a solution when challenges arise, Lenkel emphasized that ombudsmen are not empowered to make change or reverse agency decisions, and therefore the appointment would not be a panacea.
“The ombudsman doesn’t have power. We say we have the power of persuasion. And sometimes I get a company, usually small companies that come and they argue their case, they want to show me how aggrieved they’ve been and perhaps think that I am going to say, ‘Oh yes, that’s terrible. Let’s get that fixed right away.” [When] that is not what we do,” she said.
“We can help get their point across if it’s not been clearly stated. We can help them get their voices heard. But we have no particular power. We try to work with other groups, whatever office is involved, and persuade them to listen again with the company. … We just try to get people to talk and listen to both sides,” she said.
Alison Bodor, a member of the Alliance’s board of directors and president and CEO of the American Frozen Food Institute, recognized the limitations of an agency ombudsman during the webinar, but added “there’s an opportunity across all of the agencies for us all to work better and [communicate better].”
In conversation with Lenkel, Bodor added that “the opportunity with the new Deputy Commissioner of Human Foods is to have greater communication with stakeholders and transparency and collaboration. And hopefully, an Office of the Ombudsman will help spur that forward.”