Catching up with FoodNavigator-USA at the IFT show, Dr Alison Van Eenennaam said that the salmon was being judged by a regulatory regime that is triggered by the process used to produce an animal for human consumption, rather than the unique characteristics and attributes of the animal itself.
This fish is no different to a fast-growing chicken

And this makes little sense from a food safety perspective, said Dr Van Eenennaam, who works in the department of animal science at the University of California, Davis.
“The trigger for regulatory review should be the novelty of the introduced traits, not the nature of the process to get there.”
“This fish is no different to a fast-growing chicken - it’s just a fast-growing fish and there is nothing dangerous about the protein being used [the AquAdvantage salmon includes a gene from the faster-growing Pacific Chinook salmon]. It also has sustainability benefits because the fish get to market faster and eat less.
“If I made the same fish using conventional selection process with elevated levels of growth hormones I’d have no regulatory hurdles to go through”, added Dr Van Eenennaam, who is a member of the FDA’s Veterinary Medicine Animal Committee, an independent expert panel that reviews new animal drug applications at the agency’s request.
“The regulatory process should be consistent across products that have equivalent levels of risk.”
It’s been such a protracted regulatory process that what we’re seeing is investment moving offshore
AquaBounty first submitted its investigational new animal drug application in 1995 and has still not had a ‘yes’ or a ‘no’ from the FDA - which gave it the provisional thumbs up in 2010, she said.
And its experience is likely to put off all but the most patient – or fabulously wealthy – investors in GE animals in future, she predicted.
“It’s been such a protracted regulatory process that what we’re seeing is investment moving offshore.”
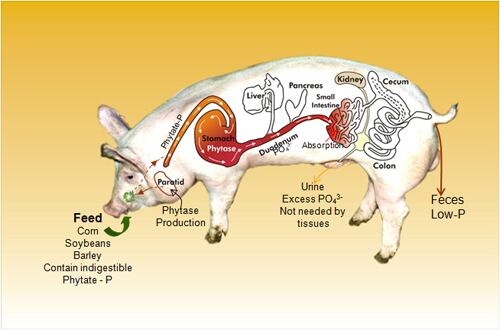
Genetic engineering of animals could have significant benefits, from cows resistant to mastitis (which is costly commercially and environmentally as well as painful for the animal), to ‘enviro-pigs’ that produce lower levels of fecal phosphorus, which are more environmentally friendly because this reduces the risk of the phosphorus polluting water sources, she said.
Click here to read more about the regulatory process for assessing GE animals.
Click here for our latest coverage on genetically engineered animals and crops.
