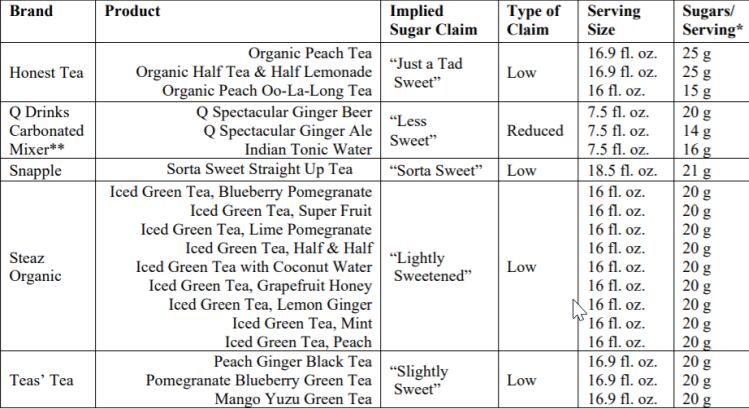
In a letter to the FDA dated January 9, the CSPI highlights 19 products from five brands (Snapple, Steaz Organic, Honest Tea, Q Drinks, and Teas’ Tea) using terms such as ‘lightly sweetened,’ ‘sorta sweet,’ ‘slightly sweet,’ ‘just a tad sweet,’ and ‘less sweet,’ to describe products that contain 14-25g added sugar per serving (up to half of the new 50g daily value).
These products – according to the CSPI – violate FDA’s regulation of nutrient content claims, and its general prohibition against labeling that is false or misleading.
There is no legal definition of ‘low sugar’ or ‘low in added sugar’
While there is no legal definition of ‘low sugar’ (the FDA only sets conditions of use for ‘reduced/less/lower sugar’ nutrient content claims, which are permitted on products containing at least 25% less sugar per reference amount customarily consumed than an appropriate reference food), it has issued guidance (p90) stressing that the term ‘low sugars’ is "not defined," and “may not be used.”
Terms such as ‘lightly sweetened,’ ‘sorta sweet,’ ‘slightly sweet,’ and ‘just a tad sweet’ are basically synonyms for the prohibited term 'low sugars,' claimed CSPI deputy director of regulatory affairs Sarah Sorscher (although some commentators argue that phrases such as a 'tad sweet' are subjective, and are indicative of a flavor profile rather than serving as a straight synonym for 'low sugar').
"FDA hasn’t defined 'low sugar' and that means companies can’t make 'low sugar' claims," Sorscher told FoodNavigator-USA.
"They also can’t make implied low sugar claims like 'lightly sweetened.' These claims are not authorized and they can be misleading, especially when they’re used on sugary products. Under the Nutrition Labeling Education Act, nutrient content claims have to be authorized by the FDA. That means 'low sugar' claims and synonyms can’t be made at all until they’ve been defined by the agenc."
Meanwhile, terms such as ‘less sweet’ are implied nutrient content claims because they are synonyms for ‘reduced sugar,’ and should not be permitted unless the products meet the criteria for a reduced sugar product, argues the CSPI.
“CSPI respectfully urges the FDA to take enforcement action against the manufacturers identified in this letter for making implied ‘low sugar’ or ‘reduced sugar’ claims unauthorized by current regulation, as well as any other manufacturers making similar claims that the agency may identify."
CSPI: FDA should set criteria for new 'low sugar' and 'low added sugar' nutrient content claims
The CSPI also urged the FDA "to move expeditiously to issue regulations authorizing ‘low added sugar’ claims to be made on products that are truly low in added sugars. In authorizing such claims, we encourage the agency to develop a per-RACC threshold similar to that which is used for other ‘low’ nutrient content claims, and to consider setting it no higher than 5% of the [50g] daily value [which would be 2.5g] or 3g per serving.”
According to Sorscher: "The rationale for this [proposed update to the] law is to avoid precisely the type of situation we are seeing here, where the food industry calls something 'low,' nutrition experts call the same thing 'high,' and consumers are left confused about what they’re eating."

What do the courts say?
The CSPI's letter follows a flurry of lawsuits highlighting the risks facing manufacturers using terms such as ‘lightly sweetened’ or other unregulated terms such as ‘nutritious’ or ‘wholesome’ on products plaintiffs argue contain high amounts of sugar.
Given that the FDA has not (yet) defined ‘low sugar,’ these cases come down to subjective arguments about whether ‘reasonable consumers’ feel misled, however.

Most recently, Kellogg agreed to settle a class action lawsuit alleging it falsely advertised some ‘lightly sweetened’ cereals as healthy and nutritious when they are also high in sugar. As part of the settlement, it also agreed to remove the phrase ‘Lightly Sweetened’ from Frosted Mini-Wheats and Smart Start for at least three years.
However, a similar case against General Mills was dismissed by a different judge in the same district in northern California, who argued that consumers “cannot plausibly claim to be misled about the sugar content of their cereal purchases because defendant provided them with all truthful and required objective facts about its products, on both the side panel of ingredients and the front of the products’ labeling.” Meanwhile, a near identical case vs Clif Bar was allowed to proceed.
CSPI: The FDA still needs to update the definition of 'healthy'
Sorscher at the CSPI added: "Some of those cases involved misleading claims like 'lightly sweetened' on sugary products. But the decisions often hinged not on defining 'low sugar,' but on the issue of whether various claims that imply a product is healthful ('nutritious,' 'wholesome,' etc.) are misleading when used on a product that is actually quite high in added sugar.
"Defining 'low added sugar' will make it harder for food companies to make some of these claims on sugary products, but won’t resolve all the issues. The FDA still needs to update the definition of 'healthy,' for example, to address some of the other misleading claims being made on sugary products."
Right now, how these cases will play out “depends on who the judge is and how that judge sees the issues,” said Dale Giali, partner in the LA office of law firm Mayer Brown (which is not involved in the Kellogg case) who has represented dozens of food manufacturers in false advertising lawsuits.
The FDA has set a daily reference value of 50g for added sugar, or 10% of calories based on a 2,000-calorie diet to be included on the new-look Nutrition Facts panel. It does not define ‘high’ or ‘low’ sugar, and only sets conditions of use for ‘reduced/less/lower sugar’ claims.