A non-cariogenic, low-glycemic rare sugar with 92% of the sweetness of sucrose, but only 38% of the calories (1.5cals/g), tagatose is found naturally in a variety of foods, but is produced on a commercial scale via a complex process typically starting with lactose (milk sugar) that gives it a price tag beyond the reach of most food manufacturers.
Bonumose – which has patented an alternative low-cost production method it claims could catapult tagatose from a niche to a mainstream sweetener – is one of several firms, including Unilever, Hershey, and General Mills, that have urged the FDA to rethink how tagatose is labeled.
Confidence levels were high that changes could be afoot for tagatose after the FDA exempted fellow rare sugar allulose from total and added sugars labeling in spring 2019 (a move that prompted a surge of interest in the sweetener) and then issued a request for comment on whether it should also rethink its approach to labeling other sugars metabolized differently than sucrose.
In its request for comment, the FDA said it was aware that rare sugars such as tagatose do not have the "same effects in the body as traditional sugars" and added that sugars that "provided fewer calories, that are not associated with dental caries, and that result in a lower glycemic and insulinemic response than other sugars" could help manufacturers better meet consumer needs.
However, in a letter* sent to Bonumose this week in response to its citizen petition calling for tagatose to be treated like allulose on food labels, the FDA said they should be treated differently because the caloric contribution of tagatose “is significantly higher than allulose.”
FDA: The caloric contribution of tagatose ‘is significantly higher than allulose’
The FDA acknowledged that Bonumose had provided evidence supporting the benefits of tagatose in multiple areas from improved glycemic control and prebiotic effects to reduced risk of dental caries. However, it said, calories formed “the basis for our decision here.”
At 1.5 calories per gram, tagatose has far fewer calories than sucrose (4 calories/gram), but far more than allulose (0.4cals/gram), said the FDA: “The amount of empty calories to the diet provided by D-tagatose could be significantly greater than the amount provided by, for example, allulose or non-nutritive sweeteners.”
The FDA does not reference other rare sugars in its letter, but its decision likely does not bode well for those hoping for a change in the labeling of isomaltulose, given that it has the same number of calories per gram as sucrose (4cal/g) despite its non-glycemic, tooth-friendly credentials.
Bonumose has developed a production process for tagatose (which is typically made from lactose) “with an extremely high yield, that’s fully-plant based and low cost,” claims CEO Ed Rogers: “It starts with starch and then maltodextrin and we can go straight to tagatose.”
The process, he claims, could lower the price tag to below that of most sugar alcohols, and potentially even achieve cost-parity with HFCS-55 if produced at mass scale.
Bonumose: ‘Those calories are not empty calories, despite what the letter says’
Ed Rogers, CEO at Bonumose, which is gearing up to start production of tagatose at a new facility in Virginia this summer, told FoodNavigator-USA that, “Those calories are not ‘empty calories,’ because they are due to tagatose feeding good gut bacteria the same way fiber does. The good gut bacteria consume tagatose and product short chain fatty acids, which are critical for gut health. The SFCAs have some caloric value, hence they count toward tagatose."
He added: "Tagatose has a better claim to being a healthy sugar, because it contributes positively to many aspects of health, most notably gut health. Basing the decision on calories is arbitrary and biased."
In a press release lashing out at the FDA’s “convoluted, contradictory, and illogical letter,” Bonumose wrote: “Tagatose has positive health benefits. Tagatose not only does not cause tooth decay, but it even breaks up dental plaque, meaning tagatose is suitable for toothpaste and mouthwash, and is long-recognized by U.S. and European law for a dental health claim.”
As for blood glucose, it said: “tagatose not only does not increase blood glucose levels, but also has the effect of moderating sugar spikes caused by other ingredients, and under European law can carry a blood glucose lowering claim.”
Finally, it noted, “tagatose feeds beneficial bacteria in the gut, which makes it healthy for the gut microbiome. In fact, the only calories present in tagatose are predominantly due to tagatose acting like soluble dietary fiber** in the large intestine.”
Hershey: 'Disappointed... we believe the petition was well-crafted'
Hershey – which invested in Bonumose last year via its C7 Ventures arm and is collaborating with the company to develop better for you products – told FoodNavigator-USA that it was "disappointed by the FDA’s response to the petition." It explained that it believed "the petition was well-crafted and founded on sound science and will continue to study the FDA’s response."
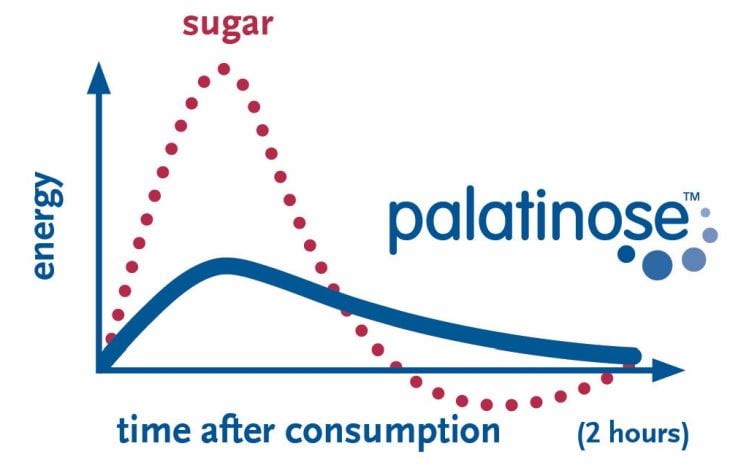
BENEO: Focusing on calories alone is too narrow
BENEO, which makes isomaltulose under the Palatinose brand, told us: "The focus on energy density/calorie counting only in approaching (new) ingredients is too narrow in BENEO’s view. While counting calories is one important aspect in weight management, it is surely not the only one.
"Diet-related high blood sugar levels are leading to a metabolic situation that inhibits fat burning and promotes fat storage and thus contributes to weight gain. Low blood sugar levels that can be achieved by the right choice of food/food ingredients are improving this situation by supporting a healthy weight. This has been demonstrated with isomaltulose in a human intervention study.
"Advantages of balanced blood glucose levels do not stop with weight management. Low glycemic food helps to avoid or delay the onset of pre-diabetes and diabetes. As such other aspects than the energy value of a food/food ingredient are relevant for a large part of the population and should not be ignored."
*In the May 18 letter to Bonumose, which has not yet been published, the FDA also declined a request to amend regulations to permit the voluntary declaration of ‘beneficial sugars’ (defined by Bonumose as ketonic monosaccharides with beneficial physiological effects) as a separate line on the Nutrition Facts panel. “There may be some sugars that are metabolized differently than traditional sugars that are not ketonic monosaccharides,” said the FDA. “Therefore, defining a class of carbohydrates in that manner may exclude some sugars metabolized differently than traditional sugars.”
** Bonumose says there is evidence that tagatose has prebiotic effects, notably that it induces the production of the short chain fatty acid butyrate and stimulates the growth of beneficial bacteria in the large intestine. However, its citizen’s petition urging the FDA to classify tagatose as a dietary fiber was denied last summer.
Tagatose is an attractive alternative to sugar as it has bulk and sugar-like sweetness, with fewer calories and a negligible impact on blood sugar, says Bonumose CEO Ed Rogers: “It is the closest thing to being a drop-in replacement for sugar in large-scale food production equipment.”
Hershey and Unilever have backed calls for tagatose to be exempted from added and total sugar labeling, although The Center for Science in the Public Interest said it had concerns about whether sugars metabolized differently from sucrose could cause digestive issues.
"We are concerned that D-tagatose, only tested on healthy adults in small studies, can also cause gastrointestinal effects at sufficient doses in some people, particularly children who use tagatose (again, estimated to have higher exposures than adult users, on a body weight basis) and those with IBS."